|
|
Solar Methanol | ||||||
|
|||||||
|
|
Solar Methanol | ||||||
|
|||||||
| Solar Methanol | |||
|
|||
| Methanol Production | |||
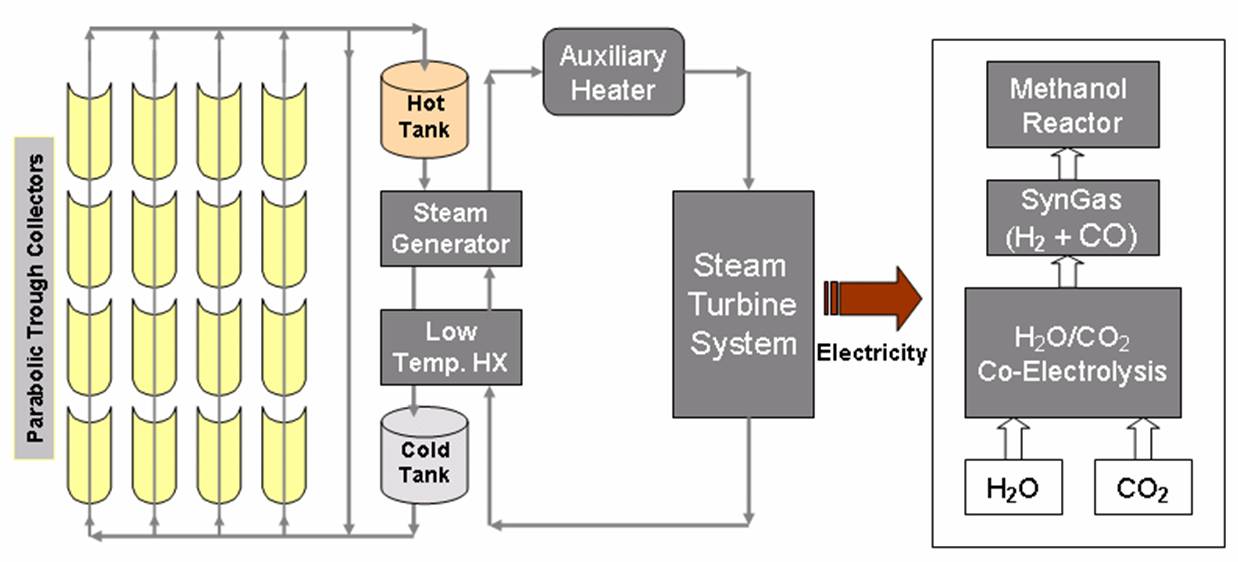
| Industrial methanol (CH3OH) is currently produced from synthesis gas (H2+ CO), which is typically derived from fossil fuel (methane). Our technology produces syngas by the co-electrolysis of H2O/CO2 streams using a solid oxide electrolyzer. Unlike the current industrial method which produces significant carbon dioxide greenhouse gas, our approach produces not such emissions. The co-electrolysis process: CO2+ H2O → H2+ CO + 3/2 O2. | |||
 |
|||
| Solar Methanol is produces by reacting the syngas at 5–10 MPa and 250 °C over a catalyst consisting of a mixture of copper, zinc oxide, and alumina: CO + 2 H2 → CH3OH. | |||
|
The energy required for the co-electrolysis of CO2+ H2O is produced from clean solar thermal energy. Parabolic-trough power technology is the most established of concentrating solar power technologies. The solar power plants use a field of Parabolic-trough collectors to supply thermal energy to a conventional power plant that generates electricity, which drives the high-temperature co-electrolysis process. |
|||
|
Solar Hydrogen
|